 5 months, 3 weeks ago
5 months, 3 weeks ago 
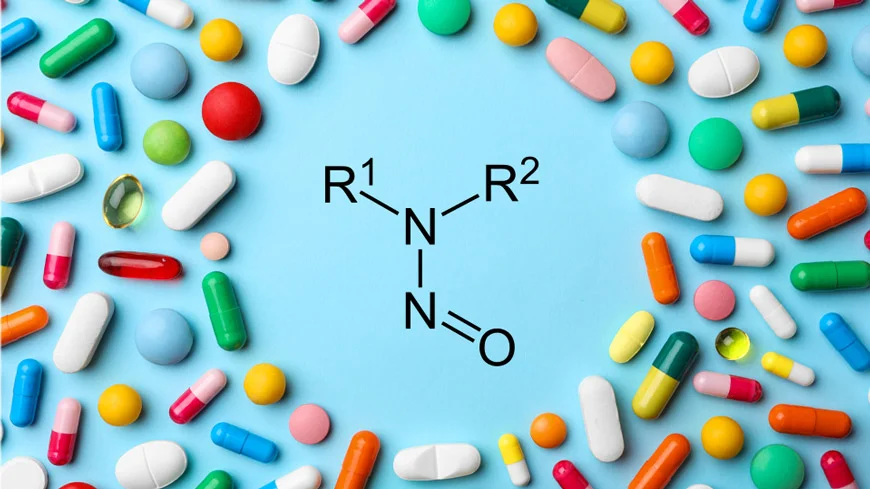
Natco Pharma USFDA 483 Flags Deficiencies in Aseptic Areas, Validation
Natco Pharma’s sterile products manufacturing facility at Rangareddy, Telengana (FEI 3004540906) was inspected by USFDA Investigators Arsen Karapetyan and Wayne







 QueriesQvents
QueriesQvents  117 Views
117 Views  0 Likes
0 Likes  0 Replies
0 Replies 


